Moderna asks Canada for extension of COVID vaccine to young children
By Trend
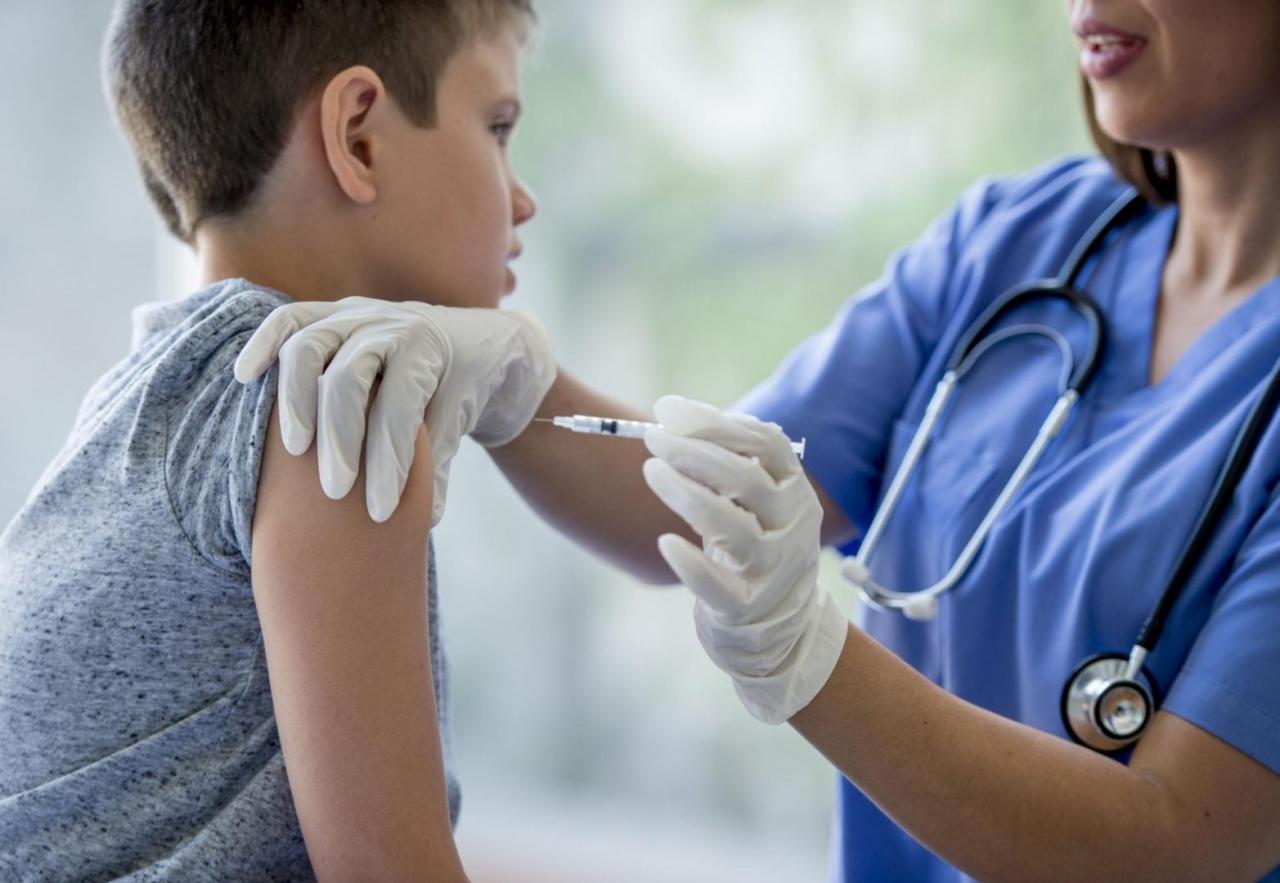
Canada is reviewing a request by Moderna to approve its COVID-19 vaccine for pediatric use in children aged 6 months to 5 years, the government and the company said on Friday, Trend reports citing Reuters.
Moderna's Canada General Manager Patricia Gauthier said at a news conference the request was filed with Canada Health on Thursday night.
Canada Health posted the application on its website on Friday and said it was under review.
Moderna said on Thursday it has asked U.S. regulators to authorize its COVID vaccine for children under the age of 6, which would make it the first shot against the coronavirus available for those 5 years old.
---
Here we are to serve you with news right now. It does not cost much, but worth your attention.
Choose to support open, independent, quality journalism and subscribe on a monthly basis.
By subscribing to our online newspaper, you can have full digital access to all news, analysis, and much more.
You can also follow AzerNEWS on Twitter @AzerNewsAz or Facebook @AzerNewsNewspaper
Thank you!
